
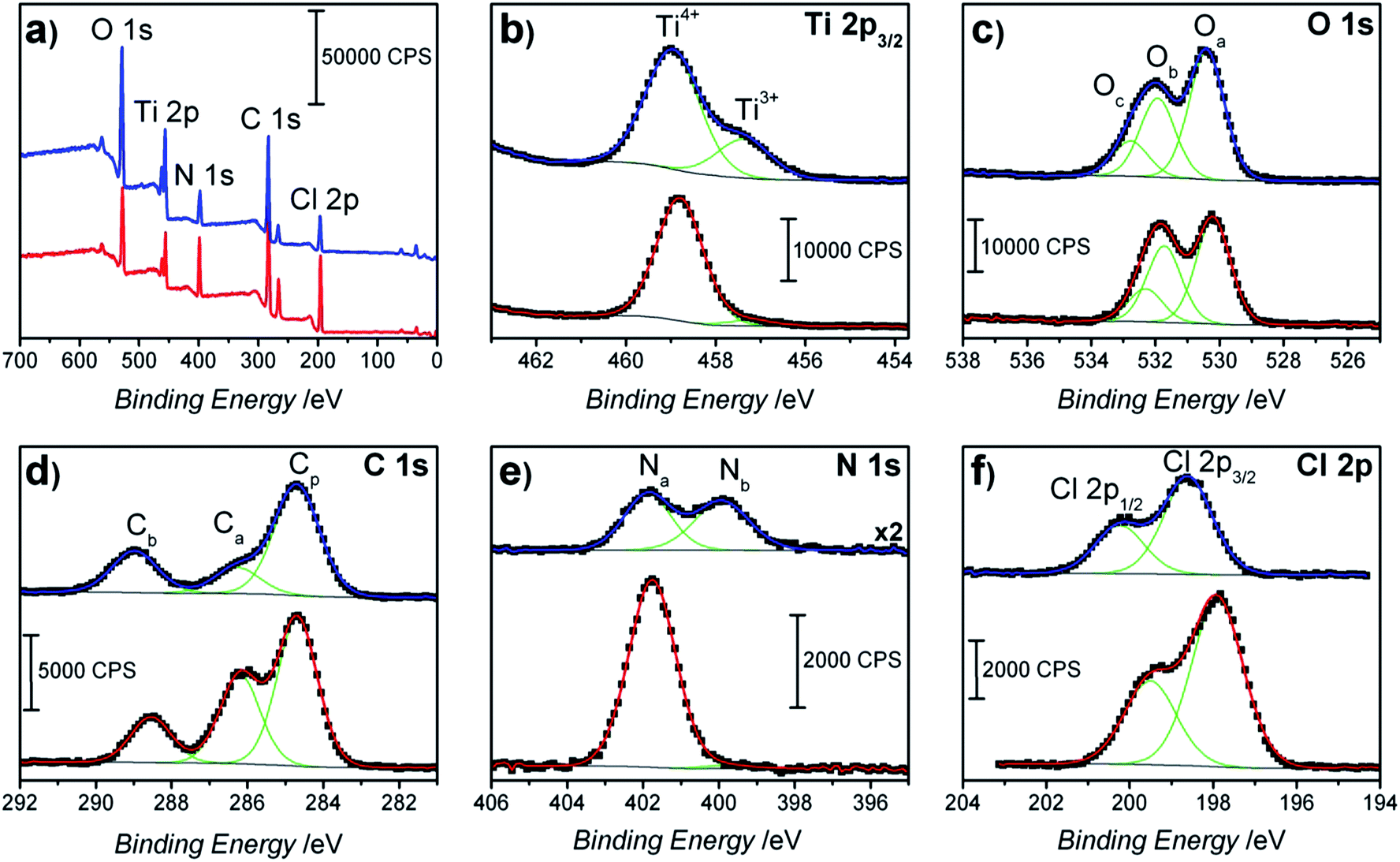
The presence of sodium, especially with the presence of chlorine and an intense hydrocarbon C1s peak can indicate contamination from manual handling, without use of gloves. PS: On a usual XPS system, a peak with up to 1.5 eV FWHM is normal, so if what you are seeing is much bellow that, maybe it's nothing. Na is a highly reactive metal and usually only sodium compounds will be observed in XPS spectra. Seems like higher concentration increases a peak with slightly lower energy (you said angle, but I'm assuming you mean Binding energy in eV?), so you see the broadening of the peak due to the appearance of a second (or third.) bonding state, and a shift for lower values due to increased fraction of the lower energy state. By deconvoluting and quantifying the fraction of each bonding state (there can be more then 2, by the way), you can see how that change is correlated to the concentration. So, like i said, i don't know much about the Chlorine 2p XPS shifts when bonded to this or that element, but from what you described, it seems that increasing the chlorine content changes the fraction of it that reacts and bonds to a certain kind of atom in your sample. This is a relatively well known procedure, you can find more about it by searching for "XPS deconvolution".
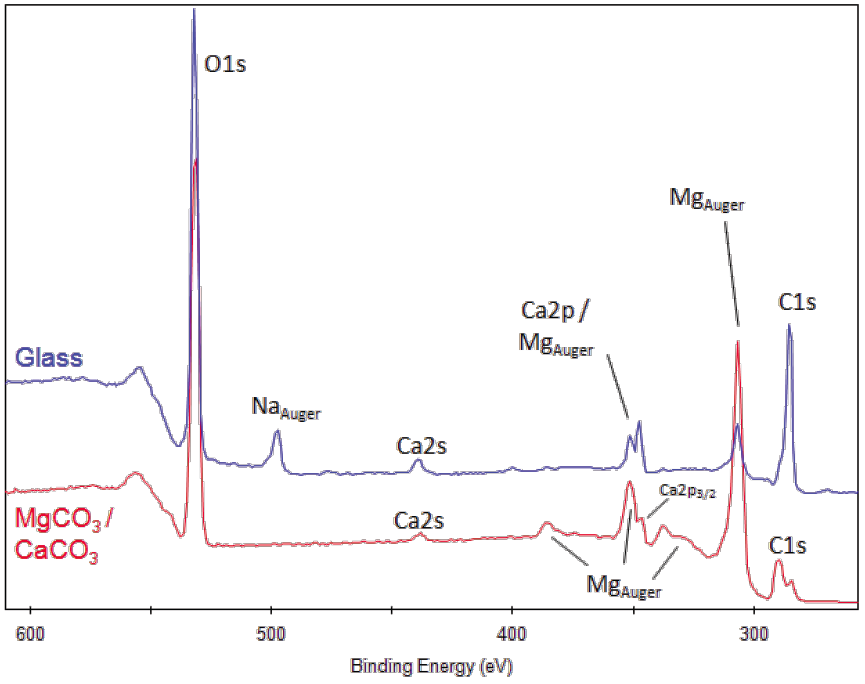
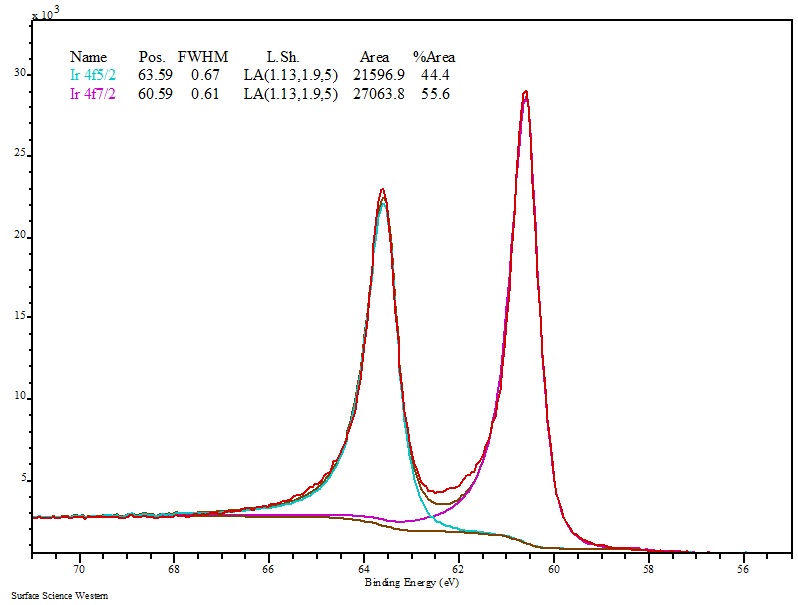
So a sample which contains carbon bonded both to carbon and to oxygen would show a broad carbon 1s peak, which could be separated by a process called deconvolution, showing two peaks inside the broad measured one. Carbon bonded to oxygen (more electronegative, pulling the molecular orbital cloud "more to it's side", so to speak) has a 1s peak energy of ~286 eV. The strong absorption peak observed at 807 cm 1 is the characteristic peak of a paradisubstituted. For example, carbon bonded to carbon has a 1s peak energy of ~284.5 eV. The peak observed at 1091 cm 1 is attributed to the stretching vibration of the SC bond. I'm not sure about Chlorine, specifically, but a wide XPS peak usually means that you have that element bonded to more then one kind of atom, which shifts the orbital's energy.
Rafael Cavalcante Cordeiro Popular answer


 0 kommentar(er)
0 kommentar(er)
